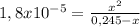Chemistry, 26.10.2019 05:43 blanca04fp
The degree to which a weak base dissociates is given by the base-ionization constant, kb. for the generic weak base, b b(aq)+h2o(l)⇌bh+(aq)+oh−(aq) this constant is given by kb=[bh+][oh−][b] strong bases will have a higher kb value. similarly, strong bases will have a higher percent ionization value. percent ionization=[oh−] equilibrium[b] initial×100% strong bases, for which kb is very large, ionize completely (100%). for weak bases, the percent ionization changes with concentration. the more dilute the solution, the greater the percent ionization. ammonia, nh3, is a weak base with a kb value of 1.8×10−5.parta what is the ph of a 0.245 m ammonia solution? partb what is the percent ionization of ammonia at this concentration?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2
You know the right answer?
The degree to which a weak base dissociates is given by the base-ionization constant, kb. for the ge...
Questions

Spanish, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

Chemistry, 12.12.2020 16:20

History, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20



English, 12.12.2020 16:20

History, 12.12.2020 16:20


History, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

Geography, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20