
Chemistry, 26.10.2019 04:43 Halessoftball
What is the molar concentration of fe2+ ion in an aqueous solution if 37.50 ml of 0.118 m kbro3 is required for complete reaction with 11.00 ml of the fe2+ solution? the net ionic equation is: 6fe2+(aq)+bro3−(aq)+6h+(aq)→6fe3+(a q)+br−(aq)+3h2o(l).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
What is the molar concentration of fe2+ ion in an aqueous solution if 37.50 ml of 0.118 m kbro3 is r...
Questions

English, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00


German, 18.11.2020 01:00

English, 18.11.2020 01:00


Computers and Technology, 18.11.2020 01:00




History, 18.11.2020 01:00

Geography, 18.11.2020 01:00


English, 18.11.2020 01:00


Engineering, 18.11.2020 01:00


Mathematics, 18.11.2020 01:00

Chemistry, 18.11.2020 01:00

Spanish, 18.11.2020 01:00

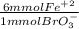 = 26.55 mmol Fe²⁺
= 26.55 mmol Fe²⁺

