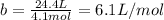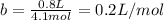Chemistry, 26.10.2019 03:43 barnhill6534
It turns out that the van dar waals constant b is equal to four times the total volume actually occupied by the molecules of a mole of gas. using this figure, calculate the fraction of the volume in a container actually occupied by ar atoms:
a) at stp
b) at 100 atm pressure and 0 degrees celsius

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
It turns out that the van dar waals constant b is equal to four times the total volume actually occu...
Questions


Physics, 07.07.2019 21:30


History, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30

History, 07.07.2019 21:30

History, 07.07.2019 21:30

Social Studies, 07.07.2019 21:30

Health, 07.07.2019 21:30




Mathematics, 07.07.2019 21:30


Mathematics, 07.07.2019 21:30



History, 07.07.2019 21:30


Mathematics, 07.07.2019 21:30

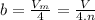 (1)
(1)