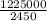Chemistry, 26.10.2019 03:43 Deavionaaaaa
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relaxes back to the ground state by emitting two photons, the first, a red photon at 700 nm, and the second, an infrared photon at 1750 nm. what is the wavelength of the absorbed photon? 500 nm 1225 nm 700 nm 1950 nm 1750 nm

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relax...
Questions

English, 31.03.2021 22:40



Geography, 31.03.2021 22:40

Arts, 31.03.2021 22:40

Mathematics, 31.03.2021 22:40

Mathematics, 31.03.2021 22:40


English, 31.03.2021 22:40

Computers and Technology, 31.03.2021 22:40

Mathematics, 31.03.2021 22:40

Business, 31.03.2021 22:40








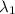 ) = 700 nm
) = 700 nm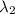 ) = 1750 nm
) = 1750 nm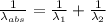
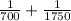
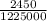
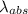 =
=