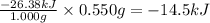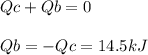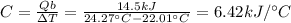Chemistry, 26.10.2019 02:43 holman9308
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. if the combustion of 0.550 g of benzoic acid causes the temperature of the calorimeter to increase from 22.01∘c to 24.27∘c, calculate the heat capacity of the calorimeter.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. i...
Questions

Mathematics, 27.03.2020 22:11

English, 27.03.2020 22:11

Mathematics, 27.03.2020 22:12




English, 27.03.2020 22:12

Mathematics, 27.03.2020 22:12

Mathematics, 27.03.2020 22:12

History, 27.03.2020 22:12

English, 27.03.2020 22:12


History, 27.03.2020 22:12



Health, 27.03.2020 22:12



Physics, 27.03.2020 22:12

Mathematics, 27.03.2020 22:12