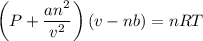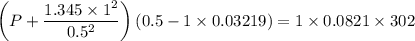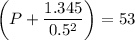Chemistry, 26.10.2019 01:43 meadowsoares7
If 1.00 mol of argon is placed in a 0.500-l container at 29.0 ∘c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 05:50
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

Chemistry, 23.06.2019 18:30
Match the following items to the correct description. 1. detainment centers for many of hitler's "undesirable" citizens, including those of the jewish race inflation 2. a form of fascist government; probably the most extreme form of tyranny first month of 1933 3. leadership taken and directed by force, often with bloodshed. an oppressive regime tyrannical government 4. financial instability brought on by price increases nazism 5. hitler became the prime minister concentration camps
Answers: 2
You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 29.0 ∘c , what is the difference between th...
Questions

Health, 16.07.2019 20:30


Mathematics, 16.07.2019 20:30

Mathematics, 16.07.2019 20:30


Social Studies, 16.07.2019 20:30

Mathematics, 16.07.2019 20:30

Arts, 16.07.2019 20:30



Biology, 16.07.2019 20:30





English, 16.07.2019 20:30

Biology, 16.07.2019 20:30

Biology, 16.07.2019 20:30