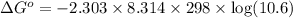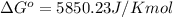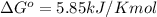Chemistry, 26.10.2019 00:43 noellelovebug1214
At 25 ∘c , the equilibrium partial pressures for the reaction were found to be pa=5.16 bar, pb=5.04 bar, pc=4.11 bar, and pd=4.85 bar . a(g)+2b(g)↽−−⇀4c(g)+d(g) what is the standard change in gibbs free energy of this reaction at 25 ∘c ? δ∘rxn= kjmol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

You know the right answer?
At 25 ∘c , the equilibrium partial pressures for the reaction were found to be pa=5.16 bar, pb=5.04...
Questions


Mathematics, 07.12.2021 03:20







Health, 07.12.2021 03:20


Biology, 07.12.2021 03:20

English, 07.12.2021 03:20

Mathematics, 07.12.2021 03:20



SAT, 07.12.2021 03:20




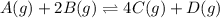
![K_p=\frac{[p_{D}]\times [p_{C}]}^4{[p_{B}]^2\times [p_{A}]}](/tpl/images/0346/8447/cda48.png)
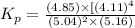
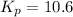
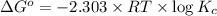
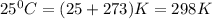
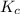 = equilibrium constant = 10.6
= equilibrium constant = 10.6