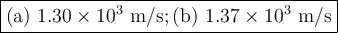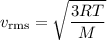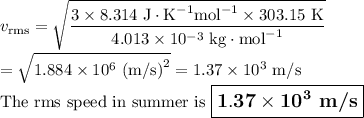Helium (he) is the lightest noble gas component of air, and xenon (xe) is the heaviest. perform the following calculations, using r = 8.314 j/(mol·k) and ℳ in kg/mol. (a) find the rms speed of he in winter (0.°c) and in summer (30.°c). enter your answers in scientific notation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
Helium (he) is the lightest noble gas component of air, and xenon (xe) is the heaviest. perform the...
Questions

Biology, 07.12.2020 21:40





Computers and Technology, 07.12.2020 21:40



English, 07.12.2020 21:40

Mathematics, 07.12.2020 21:40






Mathematics, 07.12.2020 21:40


History, 07.12.2020 21:40


Mathematics, 07.12.2020 21:40