Chemistry, 25.10.2019 19:43 niceguy1997
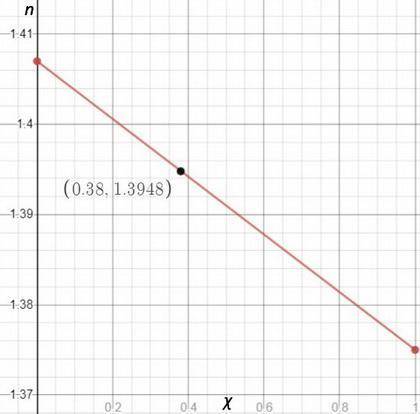
You are performing a simple distillation of roughly 50: 50 liquid solution containing two components, hexane and nonane you place 15 ml of the mixture in a round bottom flask, and then collect the distillate sequentially as four three ml samples labeled s1, s2, s3, and s4. pure hexane has a refractive index of 1.375 and pure nonane has a refractive index of 1.407. you measure a refractive index of 1.3948 for one of the four samples. assuming the refractive index varies linearly with mole fraction, estimate the mole fraction of hexane in this sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

You know the right answer?
You are performing a simple distillation of roughly 50: 50 liquid solution containing two components...
Questions

Biology, 19.03.2020 02:32


English, 19.03.2020 02:32

Mathematics, 19.03.2020 02:32



Chemistry, 19.03.2020 02:32



English, 19.03.2020 02:32




Mathematics, 19.03.2020 02:33

Mathematics, 19.03.2020 02:33




History, 19.03.2020 02:33