
Chemistry, 25.10.2019 17:43 SuperWoman9172
Asample of gas contains 0.1900 mol of co(g) and 0.1900 mol of no(g) and occupies a volume of 22.0 l. the following reaction takes place: 2co(g) + 2no(g)2co2(g) + n2(g) calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1



Chemistry, 23.06.2019 18:00
Which of the following hydrocarbons will most likely be found around cows? a. ethane b. methane c. octane d. decane
Answers: 2
You know the right answer?
Asample of gas contains 0.1900 mol of co(g) and 0.1900 mol of no(g) and occupies a volume of 22.0 l....
Questions

Mathematics, 23.01.2020 16:31

History, 23.01.2020 16:31



History, 23.01.2020 16:31

Mathematics, 23.01.2020 16:31

Mathematics, 23.01.2020 16:31


Mathematics, 23.01.2020 16:31



Health, 23.01.2020 16:31




Mathematics, 23.01.2020 16:31

History, 23.01.2020 16:31

Biology, 23.01.2020 16:31


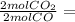 0.1900 mol CO₂
0.1900 mol CO₂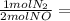 0.095 mol N₂
0.095 mol N₂

