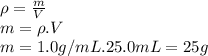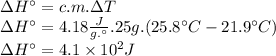Chemistry, 25.10.2019 02:43 hayleymckee
Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and water separated by a thin plastic divider. when the divider is broken, the ammonium nitrate dissolves according to the following endothermic reaction: nh4no3(> nh4 ^+(aq) + no3^-(aq). in order to measure the enthalpy change for this reaction, 1.25 g of nh4no3 is dissolved in enough water to make 25.0 ml of solution. the initial temperature is 25.8 degrees c and the final temperature (after the solid dissolves) is 21.9 degrees c. calculate the change in enthalpy for the reaction. (use 1.0g/ml as the density of the solution and 4.18 j/g . degrees c as the specific heat capacity.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and w...
Questions


Mathematics, 15.01.2021 19:50


Mathematics, 15.01.2021 19:50


Mathematics, 15.01.2021 19:50

Chemistry, 15.01.2021 19:50

Law, 15.01.2021 19:50


English, 15.01.2021 19:50

Mathematics, 15.01.2021 19:50

Mathematics, 15.01.2021 19:50


Arts, 15.01.2021 19:50



Mathematics, 15.01.2021 19:50

History, 15.01.2021 19:50