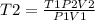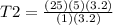Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
Calculate the final temperature (°c) of a gas if 32.0 l of the gas at 25°c and 1.00 atm is compresse...
Questions


Mathematics, 18.10.2021 20:30



Mathematics, 18.10.2021 20:30


Mathematics, 18.10.2021 20:30



Business, 18.10.2021 20:30

Social Studies, 18.10.2021 20:30





Mathematics, 18.10.2021 20:30

Chemistry, 18.10.2021 20:30


Social Studies, 18.10.2021 20:30