Chemistry, 24.10.2019 17:43 dominaricann2451
The formula of nitrogen oxide is no, of nitrogen dioxide is no_2. write a balanced equation for the reaction of nitrogen oxide with oxygen. for each (1.0) mole of no: i. how many moles of o_2 are consumed and of no_2 produced? ii. how many liters of each (state conditions)? iii. how many grams of each? d. calculate the mass of 4.55 l of hci gas at stp. e. find the molecular mass of a gas having a density of a gas having a density of 0.00481 g/ml at stp. f. write a balanced equation for the reaction you caused to occur in this assignment. solve the following problems, assume that you used a sample with a mass of 2.550 grams. i. if the sample was pure kcio_3 and it was completely decomposed into kci and o_2, fill in the following blanks: there will be moles of o_2 obtained, having a mass of grams and occupying ml at stp or ml at 29 degree c and 732 torr. ii. assume now that the sample was an unknown mixture of kcio_3 with kci and that complete decomposition yielded 182 ml of oxygen gas measured at 29 degree c and 732 torr. calculate the percent by mass of kcio_3 in the mixture. g. nitrogen gas was collected over water at 26 degree c and 755 torr. if the volume collected was 35.9l. what volume would it occupy, dry, at stp?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
The formula of nitrogen oxide is no, of nitrogen dioxide is no_2. write a balanced equation for the...
Questions


Biology, 19.01.2020 14:31

Arts, 19.01.2020 14:31

Physics, 19.01.2020 14:31

Spanish, 19.01.2020 14:31


Biology, 19.01.2020 14:31


Mathematics, 19.01.2020 14:31


English, 19.01.2020 14:31

Biology, 19.01.2020 14:31

Biology, 19.01.2020 14:31

Mathematics, 19.01.2020 14:31


Mathematics, 19.01.2020 14:31


Biology, 19.01.2020 14:31


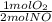 = 0,5 mol of O₂
= 0,5 mol of O₂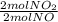 = 1 mol of NO₂
= 1 mol of NO₂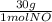 = 30g
= 30g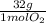 = 16g
= 16g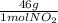 = 46g
= 46g = 7,41 g of HCl
= 7,41 g of HCl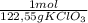 = 0,0208 moles of KClO₃
= 0,0208 moles of KClO₃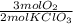 = 0,0312 moles of O₂
= 0,0312 moles of O₂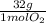 = 0,999g of O₂
= 0,999g of O₂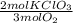 = 0,0106 mol KClO₃. In grams:
= 0,0106 mol KClO₃. In grams: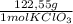 = 1,300 g of KClO₃.
= 1,300 g of KClO₃.