
Chemistry, 24.10.2019 03:00 morganlynn18
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (provides a -, the conjugate base of ha). pk a for ha is 6.23. how many moles of a - will be present in the mixture after addition of 0.20 ml 0.010 m naoh? (suggestion: start by calculating the moles of everything in the mixture.) enter the numerical value, without units, to at least 2 significant figures. you may use e-format scientific notation, e. g., 1.23x10 -4 would be entered as 1.23e-4. (note that the acid and base do not add up to 20.00 ml -- water must be added. that does not affect how you answer the question.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (prov...
Questions

Business, 04.08.2019 14:30

Mathematics, 04.08.2019 14:30

Business, 04.08.2019 14:30

History, 04.08.2019 14:30

English, 04.08.2019 14:30




Chemistry, 04.08.2019 14:30

Social Studies, 04.08.2019 14:30


Biology, 04.08.2019 14:30



Mathematics, 04.08.2019 14:30

Geography, 04.08.2019 14:30


Biology, 04.08.2019 14:30


English, 04.08.2019 14:30

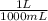 = 5x10⁻³ L of HA
= 5x10⁻³ L of HA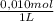 = 5x10⁻⁵ mol of HA
= 5x10⁻⁵ mol of HA

