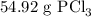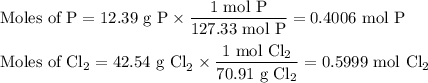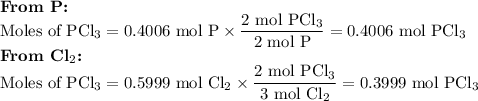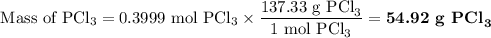Chemistry, 24.10.2019 01:00 peggycab4201
Phosphorous trichloride can be formed via a combination reaction from its elements. phosphorous is often represented by its empirical formula, p, in chemical equations, as is carbon. if 12.39 g of phosphorous is combined with 42.54 g of chlorine, what mass of phosphorous trichloride could be formed?

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2
You know the right answer?
Phosphorous trichloride can be formed via a combination reaction from its elements. phosphorous is o...
Questions


Mathematics, 17.11.2020 21:30

History, 17.11.2020 21:30


Biology, 17.11.2020 21:30

Biology, 17.11.2020 21:30

Biology, 17.11.2020 21:30






Mathematics, 17.11.2020 21:30




Mathematics, 17.11.2020 21:30


Mathematics, 17.11.2020 21:30