
Chemistry, 23.10.2019 23:00 robbinsjeffrey271
Asample of nitrosyl bromide (nobr) decomposes according to the equation 2nobr(g)⇌2no(g)+br2(g) an equilibrium mixture in a 5.00-l vessel at 100 ∘c contains 3.29 g of nobr, 3.04 g of no, and 8.10 g of br2. part apart complete calculate kc. express your answer using three significant figures. 0.116 previous answers correct part bpart complete what is the total pressure exerted by the mixture of gases? express your answer to three significant figures and include the appropriate units. 1.11 atm previous answers correct part c what was the mass of the original sample of nobr?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Asample of nitrosyl bromide (nobr) decomposes according to the equation 2nobr(g)⇌2no(g)+br2(g) an eq...
Questions



Mathematics, 10.12.2021 19:10


Mathematics, 10.12.2021 19:10

History, 10.12.2021 19:10

Computers and Technology, 10.12.2021 19:10

Business, 10.12.2021 19:10

Mathematics, 10.12.2021 19:10


Computers and Technology, 10.12.2021 19:10

Mathematics, 10.12.2021 19:10

Geography, 10.12.2021 19:10

Biology, 10.12.2021 19:10

Mathematics, 10.12.2021 19:10



Mathematics, 10.12.2021 19:10


Mathematics, 10.12.2021 19:10

![kc = \frac{[Br_{2}][NO]^2{[NOBr]^2}](/tpl/images/0343/4270/3b92b.png) (1)
(1)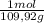 = 0,0299 moles/ 5,00L = 5,986x10⁻³M
= 0,0299 moles/ 5,00L = 5,986x10⁻³M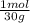 = 0,101 moles/ 5,00L = 0,0203M
= 0,101 moles/ 5,00L = 0,0203M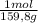 = 0,0507 moles/ 5,00L = 0,0101M
= 0,0507 moles/ 5,00L = 0,0101M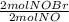 = 0,101 mol NOBr
= 0,101 mol NOBr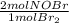 = 0,101 mol NOBr
= 0,101 mol NOBr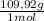 = 25,5 g of NOBr
= 25,5 g of NOBr

