
Chemistry, 23.10.2019 23:00 itzygutierrez2763
8.5 ml of a 0.53 m solution of the strong acid hcl was diluted with water to a total volume of 1000.0 ml at 30°c. calculate the following. (the kw at 30°c is 1.46 ✕ 10−14.)
(a) the concentration of h3o+ ions in the diluted hcl solution m
(b) the ph of the diluted solution (c) the poh of the diluted solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
8.5 ml of a 0.53 m solution of the strong acid hcl was diluted with water to a total volume of 1000....
Questions



Mathematics, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40


Mathematics, 13.11.2020 23:40


Social Studies, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40




Mathematics, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40

English, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40

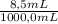 = 4,5x10⁻³ M of HCl
= 4,5x10⁻³ M of HCl


