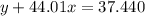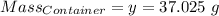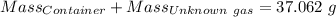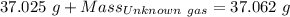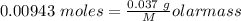Chemistry, 23.10.2019 20:30 camirialchambers17
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the same container filled with carbon dioxide at stp has a mass of 37.440 g. when filled with an unknown gas at stp, the container mass is 37.062 g. calculate the molecular weight of the unknown gas, and then state its probable identity.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the...
Questions

Chemistry, 28.09.2019 20:30

English, 28.09.2019 20:30





Chemistry, 28.09.2019 20:30

History, 28.09.2019 20:30


History, 28.09.2019 20:30

Mathematics, 28.09.2019 20:30

English, 28.09.2019 20:30


Social Studies, 28.09.2019 20:30

Mathematics, 28.09.2019 20:30

History, 28.09.2019 20:30

English, 28.09.2019 20:30

Mathematics, 28.09.2019 20:30

Health, 28.09.2019 20:30

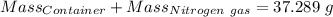
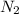 = 28.014 g/mol
= 28.014 g/mol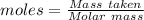
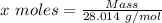
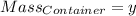
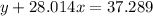
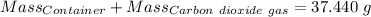
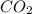 = 44.01 g/mol
= 44.01 g/mol