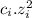Chemistry, 23.10.2019 19:50 justin20080
For each of the scenarios, determine if the ionic strength of the solution would increase, decrease, or not change. ignore any effects caused by the change in volume. if a solution of hno3 were added to a solution of koh, the ionic strength of the koh solution would: a) not change b) increase c) decrease. if a dilute solution of koh were added to a solution of cacl2 (ca(oh)2(s) is formed), the ionic strength of the cacl2 solution would : a) not change b) decrease c) increase. if a dilute solution of benzoic acid were titrated with a solution of koh, the ionic strength of the benzoic acid solution would: a) not change b) decrease c) increase. if a solution of naoh were added to a solution of iron(ii) chloride, the ionic strength of the iron(ii) chloride solution would: a) not change b) decrease c) increase.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
For each of the scenarios, determine if the ionic strength of the solution would increase, decrease,...
Questions




Physics, 07.11.2019 22:31

Social Studies, 07.11.2019 22:31

Mathematics, 07.11.2019 22:31



Mathematics, 07.11.2019 22:31


Biology, 07.11.2019 22:31



Mathematics, 07.11.2019 22:31


History, 07.11.2019 22:31

Mathematics, 07.11.2019 22:31


Chemistry, 07.11.2019 22:31