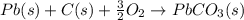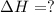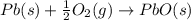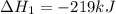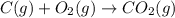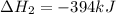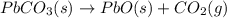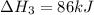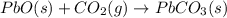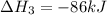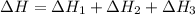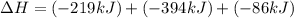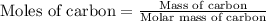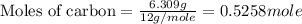Chemistry, 23.10.2019 19:30 desiiraee6265
Use the following information to calculate the amount of heat involved in the complete reaction of 6.309 of carbon to from pbco3 (s) in reaction 4. be sure to give the proper sign (positive or negative) with your answer. (1) pb(s)+1/2 o2 right arrow pbo(s) (2) c(g)+o2(g) right arrow co2(g) (3) pbco3(s)right arrow pbo(s)+co2(g) (4) pb(s)+c(s)+3/2 o2(g) right arrow pbo3(s) delta h degree rsn= -219 kj delta h degree rsn= -394 kj delta h degree rsn= 86 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1


Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2
You know the right answer?
Use the following information to calculate the amount of heat involved in the complete reaction of 6...
Questions

Mathematics, 13.02.2020 19:56

Mathematics, 13.02.2020 19:56

Mathematics, 13.02.2020 19:56






History, 13.02.2020 19:56

English, 13.02.2020 19:56


Mathematics, 13.02.2020 19:56



Mathematics, 13.02.2020 19:56





English, 13.02.2020 19:56