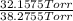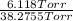Chemistry, 23.10.2019 19:20 SavgeKid7578
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour pressure of pure ccl4 is 33.85 torr at 298k. the henry’s law constant when the concentration of br2 is expressed as a mole fraction is 122.36 torr. calculate the vapour pressure of each component, the total pressure, and the composition of the vapour phase when the mole fraction of br2 is 0.050, on the assumption that the conditions of the ideal-dilute solution are satisfied at this condition

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour...
Questions





History, 12.08.2020 08:01




Business, 12.08.2020 08:01

English, 12.08.2020 08:01










Mathematics, 12.08.2020 08:01

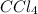 = 33.85 Torr
= 33.85 Torr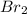 = 122.36 torr
= 122.36 torr