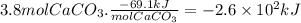Chemistry, 23.10.2019 18:00 noahdavis4650
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancient romans as mortar in stone structures. the reaction for this process is
ca(oh)2(s) + co2(g) --> caco3(s) + h2o(g) ; δh = -69.1 kj
what is the enthalpy change if 3.8 mol of calcium carbonate is formed?
(a) -18 kj
(b) -69 kj
(c) -73 kj
(d) -260 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancie...
Questions

History, 20.09.2020 04:01

English, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01






Biology, 20.09.2020 04:01

English, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Social Studies, 20.09.2020 04:01




English, 20.09.2020 04:01

Biology, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01