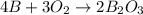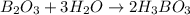Chemistry, 23.10.2019 18:30 jasmin2344
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron trioxide is then reacted with a measured quantity of water, it reacts with the water to form what is commonly known as boric acid, b(oh)3. write a balanced chemical equation for each of these processes. (use the lowest possible coefficients. omit states-of-matter in your answer.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron tr...
Questions



Social Studies, 15.10.2019 13:20


Biology, 15.10.2019 13:20

Social Studies, 15.10.2019 13:20

Mathematics, 15.10.2019 13:20


Mathematics, 15.10.2019 13:20



Mathematics, 15.10.2019 13:20







Mathematics, 15.10.2019 13:20

Chemistry, 15.10.2019 13:20