Chemistry, 23.10.2019 18:30 dannyelleparker9680
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is 21/760 16 mm hg 760/21 120/75 160 mm hg submitr

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
True or false, the three major scales used to measure earthquakes are mercalli scale, richter scale and magnitude scale
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


You know the right answer?
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of t...
Questions


English, 21.09.2019 05:00


Biology, 21.09.2019 05:00


History, 21.09.2019 05:00

Mathematics, 21.09.2019 05:00

Mathematics, 21.09.2019 05:00


Mathematics, 21.09.2019 05:00

English, 21.09.2019 05:00



Biology, 21.09.2019 05:00

Mathematics, 21.09.2019 05:00

Mathematics, 21.09.2019 05:00




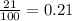
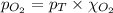
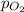 = partial pressure of oxygen = ?
= partial pressure of oxygen = ?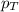 = total pressure of air = 760 mmHg
= total pressure of air = 760 mmHg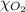 = mole fraction of oxygen = 0.21
= mole fraction of oxygen = 0.21