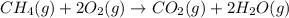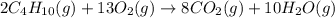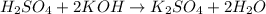Chemistry, 23.10.2019 17:50 dxnimxriee
Methane ch4 (g) reacts with oxygen gas to produce carbon dioxide and water. b) butane c4h10 (g) reacts with oxygen gas to produce carbon dioxide and water. c) an aqueous solution of sulfuric acid reacts with aqueous potassium hydroxide to produce potassium sulfate and water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
Methane ch4 (g) reacts with oxygen gas to produce carbon dioxide and water. b) butane c4h10 (g) rea...
Questions

Mathematics, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00




Mathematics, 10.11.2020 14:00


Computers and Technology, 10.11.2020 14:00


Mathematics, 10.11.2020 14:00

French, 10.11.2020 14:00



Chemistry, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00



History, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00