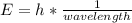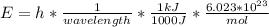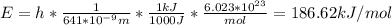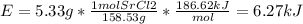Chemistry, 23.10.2019 17:00 camirialchambers17
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compound excites certain salts, which emit specific colors. strontium salts have an intense emission at 641 nm. what is the energy (in kj) of this emission for 5.33 g of the chloride salt of strontium? assume that all the heat produced is converted to emitted light. enter to 2 decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1


Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compoun...
Questions


Mathematics, 12.11.2020 06:10


Mathematics, 12.11.2020 06:10



Biology, 12.11.2020 06:10


Health, 12.11.2020 06:10


English, 12.11.2020 06:10



Physics, 12.11.2020 06:10

Arts, 12.11.2020 06:10

Mathematics, 12.11.2020 06:10



Mathematics, 12.11.2020 06:10

Mathematics, 12.11.2020 06:10