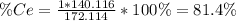Chemistry, 23.10.2019 16:50 chelseychew32
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated with excess iodate to precipitate the ce4+ as ce(io3)4. the precipitate was collected, washed well, dried, and ignited to produce 0.1848 g of ceo2 (fm 172.114). what was the weight percentage of ce (am 140.116) in the original sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2


Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated...
Questions




Biology, 28.05.2021 20:30

Mathematics, 28.05.2021 20:30


English, 28.05.2021 20:30

Mathematics, 28.05.2021 20:30



Mathematics, 28.05.2021 20:30

Mathematics, 28.05.2021 20:30

Mathematics, 28.05.2021 20:30







Business, 28.05.2021 20:30

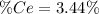
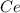 into the
into the 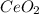 by using their respective molar masses as shown below:
by using their respective molar masses as shown below: