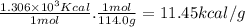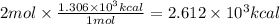Combustion reactions are exothermic. the heat of reaction for the combustion of 2-methylheptane, c8h18, is 1.306×103 kcal/mol. what is the heat of combustion for 2-methylheptane in kcal/gram? 11.45 kcal/gram how much heat will be given off if molar quantities of 2-methylheptane react according to the following equation? 2 c8h18 + 25 o216 co2 + 18 h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
Combustion reactions are exothermic. the heat of reaction for the combustion of 2-methylheptane, c8h...
Questions


Mathematics, 25.03.2021 18:50


Business, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50



French, 25.03.2021 18:50

Biology, 25.03.2021 18:50



Biology, 25.03.2021 18:50

Chemistry, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50



Arts, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50