
Chemistry, 23.10.2019 00:50 Drellibus70011
Nthe formation of chloromethane and hydrogen chloride. the overall reaction is 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) suppose that a chemist combines 295 ml295 ml of methane and 725 ml725 ml of chlorine at stp in a 2.00 l2.00 l flask. the flask is then allowed to stand at 298 k.298 k. if the reaction reaches 77.0%77.0% completion, what is the total pressure in the flask?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Nthe formation of chloromethane and hydrogen chloride. the overall reaction is 2ch4(g)+3cl2(g)⟶2ch3c...
Questions

Physics, 30.01.2020 17:03






Mathematics, 30.01.2020 17:03



Arts, 30.01.2020 17:03





Mathematics, 30.01.2020 17:03

Mathematics, 30.01.2020 17:03



Mathematics, 30.01.2020 17:03

Mathematics, 30.01.2020 17:03

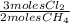 = 0,0198 moles Cl₂. That means that 0,0324-0,0198 = 0,0126 moles of Cl₂ are in excess.
= 0,0198 moles Cl₂. That means that 0,0324-0,0198 = 0,0126 moles of Cl₂ are in excess.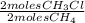 = 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-
= 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-

