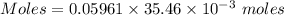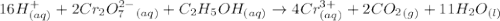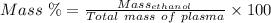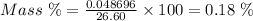Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with a potassium dichromate solution. the balanced equation is 16h (aq) 2cr2o72−(aq) c2h5oh(aq) → 4cr3 (aq) 2co2(g) 11h2o(l) if 35.46 ml of 0.05961 m cr2o72− is required to titrate 26.60 g of plasma, what is the mass percent of alcohol in the blood?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

You know the right answer?
Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with...
Questions





History, 09.07.2019 16:20

Social Studies, 09.07.2019 16:20

Social Studies, 09.07.2019 16:20

History, 09.07.2019 16:20

Social Studies, 09.07.2019 16:20


Biology, 09.07.2019 16:20


Social Studies, 09.07.2019 16:20

Chemistry, 09.07.2019 16:20

Social Studies, 09.07.2019 16:20


History, 09.07.2019 16:20


History, 09.07.2019 16:30

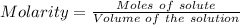
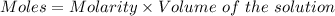
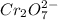 :
: