Two aqueous sulfuric acid solutions containing 20.0 wt% h2so4
(sg = 1.139) and 60.0 wt%...

Two aqueous sulfuric acid solutions containing 20.0 wt% h2so4
(sg = 1.139) and 60.0 wt% h2so4 (sg = 1.498) are mixed to form a
4.00 molar solution (sg = 1.213). taking 100kg of the 20% feed
solution as a basis, calculate the feed ratio (liters 20%
solution/liter 60% solution).

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Questions


Mathematics, 16.02.2021 19:10


History, 16.02.2021 19:10


Biology, 16.02.2021 19:10

English, 16.02.2021 19:10



Mathematics, 16.02.2021 19:10

Mathematics, 16.02.2021 19:10

Mathematics, 16.02.2021 19:10

Mathematics, 16.02.2021 19:10


Mathematics, 16.02.2021 19:10

Mathematics, 16.02.2021 19:10



Mathematics, 16.02.2021 19:10

History, 16.02.2021 19:10

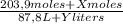 (1)
(1)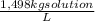 ×
×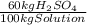 ×
×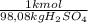 = 0,9164 kmolH₂SO₄ ≡ 916,4 moles
= 0,9164 kmolH₂SO₄ ≡ 916,4 moles

