Chemistry, 22.10.2019 23:30 hanacat6174
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following data. the heat capacity of the bomb calorimeter is 34.65 kj/k and the combustion of 1.752 g of ethanol raises the temperature of the calorimeter from 294.42 k to 295.92 k .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

You know the right answer?
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following...
Questions




Chemistry, 17.02.2022 15:20






Biology, 17.02.2022 15:30

Physics, 17.02.2022 15:30







Mathematics, 17.02.2022 15:30


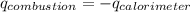
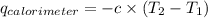
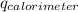 = heat released by calorimeter = ?
= heat released by calorimeter = ?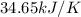
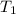 = initial temperature of calorimeter = 294.42 K
= initial temperature of calorimeter = 294.42 K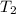 = final temperature of calorimeter = 295.92 K
= final temperature of calorimeter = 295.92 K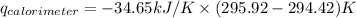
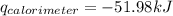
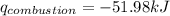
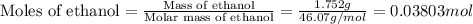
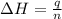
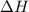 = enthalpy of combustion = ?
= enthalpy of combustion = ?