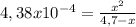For the reaction: ch3nh2(aq) + h2o(aq) ⇌ ch3nh3 +(aq) + oh- determine the change in the ph (δph) for the addition of 6.7 m ch3nh3cl salt to a 4.7 m solution of ch3nh2, kb=4.38 x 10-4 . view available hint(s) for the reaction: ch3nh2(aq) + h2o(aq) ⇌ ch3nh3 +(aq) + oh- determine the change in the ph (δph) for the addition of 6.7 m ch3nh3cl salt to a 4.7 m solution of ch3nh2, kb=4.38 x 10-4 . δph=12.66 δph=1.86 δph=10.49 δph=2.17

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
For the reaction: ch3nh2(aq) + h2o(aq) ⇌ ch3nh3 +(aq) + oh- determine the change in the ph (δph) fo...
Questions



English, 10.12.2020 18:40

Mathematics, 10.12.2020 18:40

Mathematics, 10.12.2020 18:40

Health, 10.12.2020 18:40

Spanish, 10.12.2020 18:40

Mathematics, 10.12.2020 18:40





Mathematics, 10.12.2020 18:40


English, 10.12.2020 18:40


Mathematics, 10.12.2020 18:40



Social Studies, 10.12.2020 18:40

![kb = \frac{[OH^{-}][CH_{3}NH_{3}^+]}{[CH_{3}NH_{2}]}](/tpl/images/0341/2802/33c02.png) (1)
(1)