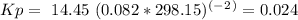Enter your answer in the provided box. a united nations toxicologist studying the properties of mustard gas, s(ch2ch2cl)2, a blistering agent used in warfare, prepares a mixture of 0.675 m scl2and 0.973 m c2h4and allows it to react at room temperature (20.0°c): scl2(g) + 2 c2h4(g) ⇌ s(ch2ch2cl)2(g) at equilibrium,[s( ch2ch2cl)2] = 0.350 m. calculate .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Enter your answer in the provided box. a united nations toxicologist studying the properties of must...
Questions



Mathematics, 26.05.2021 20:30



English, 26.05.2021 20:30


Mathematics, 26.05.2021 20:30



World Languages, 26.05.2021 20:30



Mathematics, 26.05.2021 20:30



Mathematics, 26.05.2021 20:40

Mathematics, 26.05.2021 20:40


Chemistry, 26.05.2021 20:40

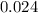
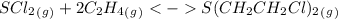
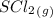 = 0.325 M
= 0.325 M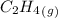 = 0.273 M
= 0.273 M 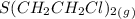 = 0.35 M
= 0.35 M![Kc=\frac{[S(CH_2CH_2Cl)_2]}{[SCl_2][C_2H_4]^2}=\frac{[0.35]}{[0.325][0.273]^2}=14.45](/tpl/images/0341/2750/de285.png)
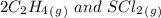 = -2
= -2