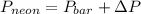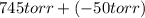Chemistry, 22.10.2019 21:00 joshuasanders7490
Aflask containing neon gas is connected to an open-endedmercury manometer. the open end is exposed to the atmosphere, wherethe prevailing pressure is 745 torr. the mercury level in the openarm is 50 mm below that in the arm connected to the flask of neon. what is the neon pressure, in torr?
a. -50 torr
b. 50 torr
c. 695 torr
d. 795 torr
e. none of these choices is correct.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
Aflask containing neon gas is connected to an open-endedmercury manometer. the open end is exposed t...
Questions

Social Studies, 27.09.2021 08:50


Mathematics, 27.09.2021 08:50



Mathematics, 27.09.2021 08:50

Mathematics, 27.09.2021 08:50


Mathematics, 27.09.2021 08:50


Physics, 27.09.2021 08:50

Mathematics, 27.09.2021 08:50



Mathematics, 27.09.2021 08:50



Social Studies, 27.09.2021 08:50

Mathematics, 27.09.2021 08:50

Mathematics, 27.09.2021 08:50

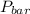 = 745 torr
= 745 torr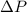 = - 50 (the value is negative because on the open side its level is lower than the side connected to the gas)
= - 50 (the value is negative because on the open side its level is lower than the side connected to the gas)