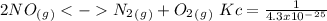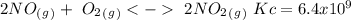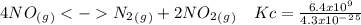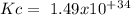Chemistry, 22.10.2019 21:00 kobiemajak
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g) + o2(g) ⇌ 2no(g) kc = 4.3 × 10−25 2no(g) + o2(g) ⇌ 2no2(g) kc = 6.4 × 109 determine the values of the equilibrium constants for the following equations at the same temperature: (a) 4no(g) ⇌ n2(g) + 2no2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g)...
Questions

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Health, 19.09.2020 01:01




Social Studies, 19.09.2020 01:01

Social Studies, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01

Computers and Technology, 19.09.2020 01:01


English, 19.09.2020 01:01

Biology, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01

Chemistry, 19.09.2020 01:01

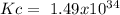
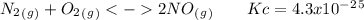
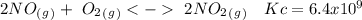
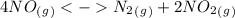
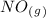 as a reactive in the target reaction and
as a reactive in the target reaction and