
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burned in excess oxygen to yield 4.23 g of co2 and 1.01 g of h2o. another sample of the same compound, of mass 4.14 g, yielded 2.11 g of so3. a third sample, of mass 5.66 g, was burned under different conditions to yield 2.27 g of hno3 as the only nitrogen containing product. determine the empirical formula of the compound.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burn...
Questions


















Mathematics, 26.12.2019 22:31

Chemistry, 26.12.2019 22:31

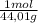 = 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt%
= 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt% 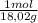 = 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%
= 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%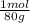 = 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%
= 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%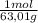 = 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%
= 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%

