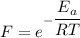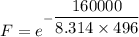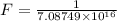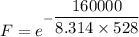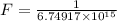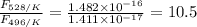The activation energy for the isomerization of methyl isonitrile is 160 kj/mol. part acalculate the fraction of methyl isonitrile molecules that have an energy of 160.0 kj or greater at 496 k .f1=part bcalculate this fraction for a temperature of 528 k .f2=part cwhat is the ratio of the fraction at 528 k to that at 496 k ? f2/f1=

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
The activation energy for the isomerization of methyl isonitrile is 160 kj/mol. part acalculate the...
Questions

History, 28.06.2019 14:00

History, 28.06.2019 14:00


Mathematics, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00



History, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00

Social Studies, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00


Mathematics, 28.06.2019 14:00

History, 28.06.2019 14:00


Mathematics, 28.06.2019 14:00


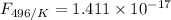
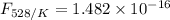
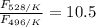
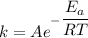
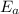 is the activation energy
is the activation energy
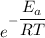 is the fraction of molecules which have value equal to or grater than activation energy.
is the fraction of molecules which have value equal to or grater than activation energy.