
Chemistry, 21.10.2019 23:30 rachelsweeney10
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then adds iron powder to the conical flask at once. then, she stirs it briskly to initiate and facilitate the reaction between copper sulfate and iron powder. while stirring continuously, vanessa observes that the blue color of copper sulfate solution is fading substantially. moreover, she also observes the deposition of a reddish-brown mass at the bottom of the flask.
finally, a stage is reached where the solution becomes pale green in color. on further stirring of the solution, there is no change in the intensity of color. a distinct reddish-brown layer is deposited in the conical flask. however, on weighing the reaction mixture, she finds that the mass of the reaction mixture remains unchanged at 215.4 g. the reddish-brown layer is then separated from the pale green solution and allowed to dry in an oven. the moisture is completely evaporated, and the dried layer is weighed. its weight is found to be 63.5 g.
what is the mass of required iron powder and the formed iron sulfate in the chemical reaction?
a
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
b
the mass of iron powder required to complete the reaction was 63.5 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
c
the mass of iron powder required to complete the reaction was 159.6 g, while the mass of iron sulfate formed in the reaction was 63.5 g.
d
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 159.6 g.
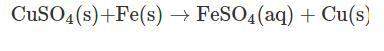

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then...
Questions


Mathematics, 05.06.2021 20:50

Mathematics, 05.06.2021 20:50


History, 05.06.2021 20:50

Computers and Technology, 05.06.2021 20:50

History, 05.06.2021 20:50

French, 05.06.2021 20:50


Mathematics, 05.06.2021 20:50

Chemistry, 05.06.2021 20:50

Mathematics, 05.06.2021 20:50



Advanced Placement (AP), 05.06.2021 20:50

Mathematics, 05.06.2021 20:50

World Languages, 05.06.2021 20:50


Mathematics, 05.06.2021 20:50




