
Chemistry, 19.10.2019 04:30 lapointayyy6388
What is the vapor pressure (in kpa) of ethanol, ch3ch2oh, over a solution which is composed of 18.00 ml of ethanol and 12.55 g of benzoic acid, c6h5cooh, at 35ºc ?
enter your number with two digits past the decimal.
•pºethanol at 35ºc = 13.693 kpa
•density of ethanol = 0.789 g/mol, molar mass of ethanol = 46.07
•molar mass of benzoic acid = 122.12 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1


Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
What is the vapor pressure (in kpa) of ethanol, ch3ch2oh, over a solution which is composed of 18.00...
Questions



English, 14.10.2020 15:01


Mathematics, 14.10.2020 15:01


Mathematics, 14.10.2020 15:01


Physics, 14.10.2020 15:01

Chemistry, 14.10.2020 15:01

Geography, 14.10.2020 15:01

Geography, 14.10.2020 15:01


Mathematics, 14.10.2020 15:01

Physics, 14.10.2020 15:01


Mathematics, 14.10.2020 15:01



Chemistry, 14.10.2020 15:01

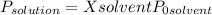 (1)
(1)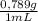 ×
×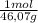 = 0,3083 mol Ethanol.
= 0,3083 mol Ethanol.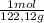 = 0,1028 mol benzoic acid.
= 0,1028 mol benzoic acid.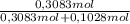 = 0,7499
= 0,7499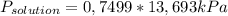 = 10,27 kPa
= 10,27 kPa

