
Chemistry, 19.10.2019 04:20 viktoria1198zz
If the percent yield for the following reaction is 80.37%, and 63.21 g of no2 are used in the reaction mixture, how many grams of nitric acid, hno3(aq), are produced in the experiment? 3 no2(g) + h2o(l) → 2 hno3(aq) + no(g) molar mass no2 = 46.01 g/mol and hno3 = 63.01 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
If the percent yield for the following reaction is 80.37%, and 63.21 g of no2 are used in the reacti...
Questions



Chemistry, 10.11.2020 23:10

Spanish, 10.11.2020 23:10

English, 10.11.2020 23:10

History, 10.11.2020 23:10

Mathematics, 10.11.2020 23:10


Biology, 10.11.2020 23:10

Mathematics, 10.11.2020 23:10


History, 10.11.2020 23:10

Mathematics, 10.11.2020 23:10


Mathematics, 10.11.2020 23:10

World Languages, 10.11.2020 23:10


English, 10.11.2020 23:10


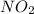 is 46.01 g/mol and mass of
is 46.01 g/mol and mass of 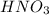 is 63.01 g/mol.
is 63.01 g/mol.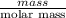
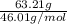
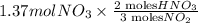
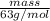
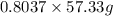
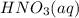 , are produced in the experiment.
, are produced in the experiment.

