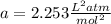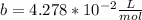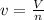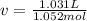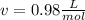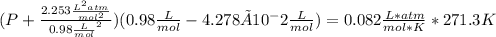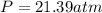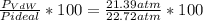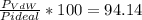1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3 k should exert a pressure of 22.72 atm. by what percent does the pressure calculated using the van der waals' equation differ from the ideal pressure? for ch4 gas,
a = 2.253 l2atm/mol2 and
b = 4.278×10-2 l/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3...
Questions



Biology, 07.04.2020 17:04


Mathematics, 07.04.2020 17:04




Biology, 07.04.2020 17:04

Mathematics, 07.04.2020 17:04

Social Studies, 07.04.2020 17:04

English, 07.04.2020 17:05

Computers and Technology, 07.04.2020 17:05