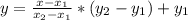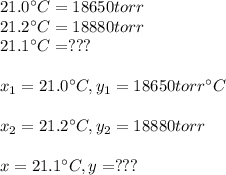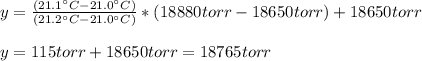Chemistry, 19.10.2019 02:20 mikaelalcool49
The vapor pressure of water at 21.0 oc is 18.650 torr and the vapor pressure of water at 21.2 oc is 18.880 torr. how can you find vapor pressure of water at 21.1 oc? explain your reasoning and give your determination of the vapor pressure of water at 21.1 oc.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
The vapor pressure of water at 21.0 oc is 18.650 torr and the vapor pressure of water at 21.2 oc is...
Questions

Mathematics, 30.07.2019 23:30






Biology, 30.07.2019 23:30

Biology, 30.07.2019 23:30



Biology, 30.07.2019 23:30






History, 30.07.2019 23:30

Physics, 30.07.2019 23:30


Mathematics, 30.07.2019 23:30