
Chemistry, 19.10.2019 01:00 adiaripley6408
The energy of an electrostatic interaction between two charged atoms is dependent on the charges on the atoms, the distance between them, and the dielectric constant of the solvent. for example, the strength of a weak acid (ka, acid dissociation constant) depends on the strength of the electrostatic interaction between a negatively charged carboxylic acid group and a proton. the solvent dielectric constant has a large influence on the pka for weak acids. select the statements that correctly explain the influence of two solvents, water and hexane, on the pka of acetic acid.
in water, the association of ch3coo– and h will be favored because charge-charge interactions are stronger in water.
nonpolar solvents like hexane will weaken charge-charge interactions resulting in more dissociation of h .
the pka will be higher in hexane.
hexane cannot affect the pka of acetic acid since hexane cannot donate or accept h .
more h will dissociate in water because the dielectric constant is higher.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2


Chemistry, 23.06.2019 16:00
Instructions: the table below explains the average rate at which some geologic processes occur. calculate the amount of sea level change, erosion, and uplift for 100,000 years, 1,000,000 years, and 10,000,000 years. remember, 100 cm = 1 m. fill out the table completely and answer the below questions in complete sentences. for with completing the table, use this video: if you need directions on how to submit your assignment, click on the link due by sunday at midnight for full credit. if submitted late, you will receive a 30% grade deduction. check your pacing guide for assignment dates. process rate per 1,000 years after 100,000 years after 1,000,000 years after 10,000,000 years sea level changes 10 m 1000 10000 100000 regional erosion 2 m 200 2000 20000 uplift 10 cm 1. what is the fastest process, sea level changes, erosion, or uplift? 2. what is the slowest process, seal level changes, erosion, or uplift?
Answers: 3
You know the right answer?
The energy of an electrostatic interaction between two charged atoms is dependent on the charges on...
Questions


History, 12.12.2020 16:50



History, 12.12.2020 16:50

Biology, 12.12.2020 16:50


Biology, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

English, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50





Arts, 12.12.2020 16:50


Computers and Technology, 12.12.2020 16:50

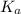 will be low , hence
will be low , hence 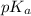 will be high.
will be high. 

