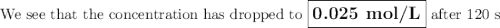Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
The decomposition of n2o5(g) —> no2(g) + no3(g) proceeds as a first order reaction with a half l...
Questions


Biology, 27.10.2020 09:30

Mathematics, 27.10.2020 09:30



Physics, 27.10.2020 09:40


Chemistry, 27.10.2020 09:40

Social Studies, 27.10.2020 09:40



SAT, 27.10.2020 09:40

Advanced Placement (AP), 27.10.2020 09:40



English, 27.10.2020 09:40

SAT, 27.10.2020 09:40



Mathematics, 27.10.2020 09:40

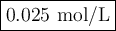
![\begin{array}{crcc}\textbf{No. of} && \textbf{Fraction} & \\\textbf{half-lives} & \textbf{t/s} & \textbf{remaining} &\rm \mathbf{{[N_{2}O_{5}] /(mol/L)}}\\0 & 0 & 1 & 0.400\\1 & 30.0 & 1/2 & 0.200\\2 & 60.0 & 1/4 & 0.100\\3 & 90.0 &1/8 & 0.050\\4 & 120.0 & 1/16 & 0.025\\5& 150.0 & 1/32 & 0.012\\\end{array}](/tpl/images/0332/8503/2bb43.png)