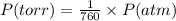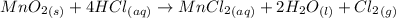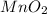Chemistry, 18.10.2019 21:00 lululoveee586
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, hcl(aq) , as described by the chemical equation mno2(s)+4hcl(aq)⟶mncl2(aq)+2h2o(l)+ cl2(g) how much mno2(s) should be added to excess hcl(aq) to obtain 255 ml cl2(g) at 25 °c and 725 torr ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions

Mathematics, 05.11.2020 14:00




English, 05.11.2020 14:00

Health, 05.11.2020 14:00

Mathematics, 05.11.2020 14:00


Mathematics, 05.11.2020 14:00




History, 05.11.2020 14:00


Chemistry, 05.11.2020 14:00

Mathematics, 05.11.2020 14:00

Physics, 05.11.2020 14:00

Chemistry, 05.11.2020 14:00