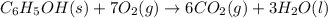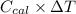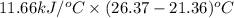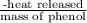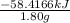Chemistry, 18.10.2019 20:00 kindaconfuseing
A1.800-g sample of solid phenol (c6h5oh(s)) was burned in a bomb calorimeter, which has a total heat capacity of 11.66 kj/∘c. the temperature of the calorimeter plus its contents increased from 21.36∘c to 26.37∘c.
(a)-write a balanced chemical equation for the reaction that takes place in the bomb calorimeter. express you answer as a chemical equation including phases. water vapor, h2o(g), will initially form as part of this reaction. however, the temperature of all substances will eventually equilibrate in the bomb calorimeter (to 26.37∘c), which signifies that water and carbon dioxide, h2o(l) and co2(g), are the eventual products of a combustion reaction in a bomb calorimeter.
(b)- what is the heat of combustion per gram of phenol?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1


Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
A1.800-g sample of solid phenol (c6h5oh(s)) was burned in a bomb calorimeter, which has a total heat...
Questions

Chemistry, 05.02.2020 00:02

Social Studies, 05.02.2020 00:02


Mathematics, 05.02.2020 00:02

Social Studies, 05.02.2020 00:02




Mathematics, 05.02.2020 00:02

English, 05.02.2020 00:02



Mathematics, 05.02.2020 00:03

Mathematics, 05.02.2020 00:03


History, 05.02.2020 00:40

English, 05.02.2020 00:40


Mathematics, 05.02.2020 00:40