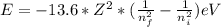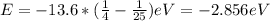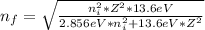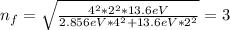Chemistry, 18.10.2019 18:30 gyexisromero10
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted. if an excited electron in an he+ ion falls from =4, which energy level must it fall to ) for blue light of a similar wavelength to be emitted?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

You know the right answer?
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted....
Questions


English, 09.11.2019 17:31


English, 09.11.2019 17:31



English, 09.11.2019 17:31

Biology, 09.11.2019 17:31

History, 09.11.2019 17:31



Mathematics, 09.11.2019 18:31


Mathematics, 09.11.2019 18:31




Mathematics, 09.11.2019 18:31