
Chemistry, 18.10.2019 01:30 vanessam16
Question 8 of 8> 0 attempt 2 a chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1284 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrates it with the standard solution. the endpoint was reached after the addition of 19.15 ml of the standard solution. what is the concentration of the original sulfuric acid solution? concentration: 025

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1
You know the right answer?
Question 8 of 8> 0 attempt 2 a chemist needs to determine the concentration of a sulfuric acid s...
Questions

Mathematics, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30

English, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30


Mathematics, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30


Mathematics, 05.03.2021 22:30





Social Studies, 05.03.2021 22:30

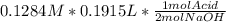 = 0.01229 mol H₂SO₄.
= 0.01229 mol H₂SO₄.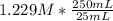 = 12.29 M
= 12.29 M

