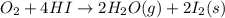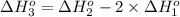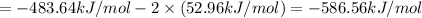Chemistry, 18.10.2019 03:30 lizchavarria863
9. calculate the standard enthalpy of the 3rd reaction using the given data: a, h° = +52.96 kj/mol afh= -483.64 kj/mol h2s)+i202 hi 2 h2g)+o2ig)2 h2o() 4 hig+o2lg)2 12)+2 h2o(e (1) (2) (3) a, h° =? 10. calculate the internal energy change for reaction (3) in the previous problem.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
9. calculate the standard enthalpy of the 3rd reaction using the given data: a, h° = +52.96 kj/mol...
Questions





Mathematics, 26.02.2020 04:34





English, 26.02.2020 04:34

Mathematics, 26.02.2020 04:34









Computers and Technology, 26.02.2020 04:35

 ...[1]
...[1]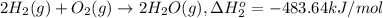 ...[2]
...[2]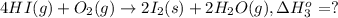 ..[3]
..[3]